Featured Post
Dalton's Law
- Get link
- X
- Other Apps
It is only an approximation for real gases. It holds true only at very low pressures.
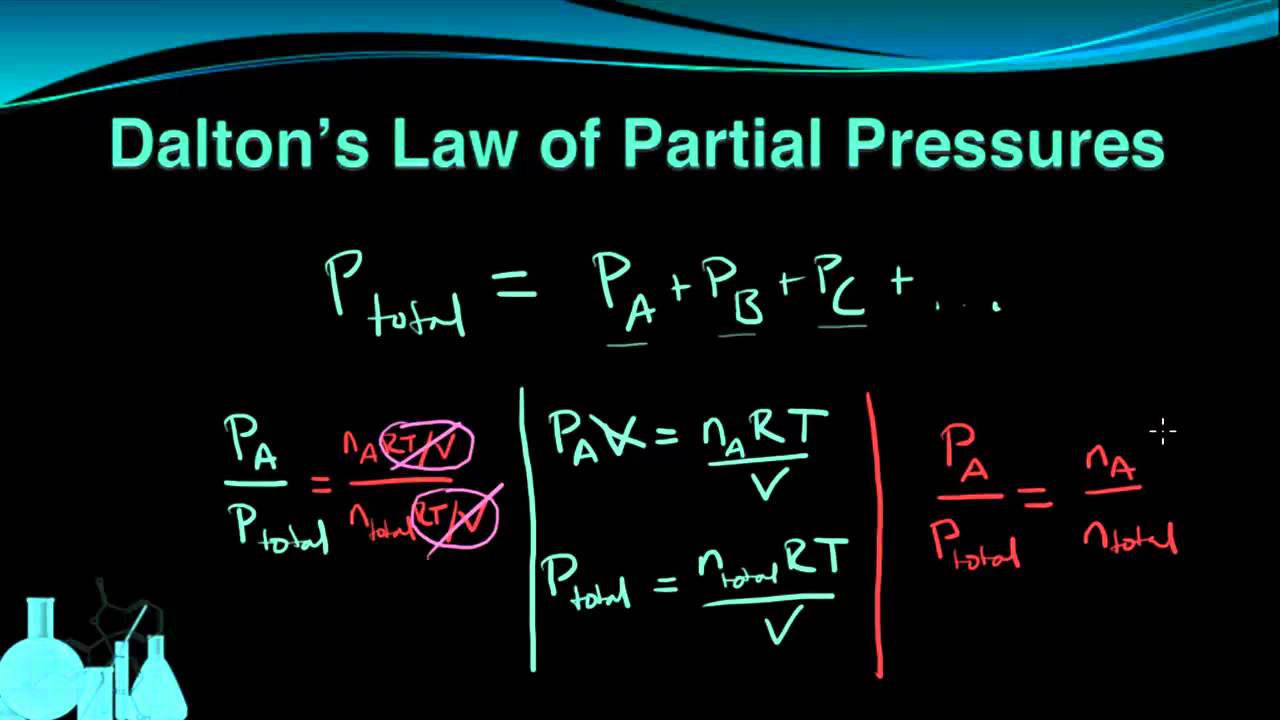
Chemistry 7 6 Dalton S Law Of Partial Pressures Dalton S Law Chemistry Dalton
Updated September 09 2019.

Dalton's law. Daltons Law is especially important in atmospheric studies. Daltons law the statement that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the individual component gases. Miller-Keane Encyclopedia and Dictionary of Medicine Nursing and Allied Health Seventh Edition.
The first part of his theory states that all matter is made of atoms which are indivisible. The pressure of a gas in a mixture equals the pressure it would exert if it occupied the same volume alone at the same temperature. Rearranging the ideal gas equation to solve for we get.
In chemistry and physics Daltons law also called Daltons law of partial pressures states that in a mixture of non-reacting gases the total pressure exerted is equal to the sum of the partial pressures of the individual gases. So the pressure exerted by dry air and pressure exerted by water vapour is known as aqueos tension. Dalton based his theory on the law of conservation of mass and the law of constant composition.
Daltons law formula states that the total pressure P Total of the mixture of gases is equal to the sum of partial pressure of individual gases in the mixture. The partial pressure is the pressure that each gas would exert if it alone occupied the volume of the mixture at the same temperature. We can also calculate the partial pressure of hydrogen in this problem using Daltons law of partial pressures which will be discussed in the next section.
Additionally who created Daltons law. Daltons law also called Daltons law of partial pressures states that The total pressure exerted by a mixture of gases is equal to the sum of the pressures of each of the different gases making up the mixture each gas acting as if it alone were present and occupied the total volume. Daltons Laws are as follows.
2003 by Saunders an imprint of Elsevier Inc. The deviation from the law increases with increasing pressure. The second part of the theory says all atoms of a given element are identical in mass and properties.
Answer 1 of 2. Daltons atomic theory was a scientific theory on the nature of matter put forward by the English physicist and chemist John Dalton in the year 1808. The atmosphere is made up principally of nitrogen oxygen carbon dioxide and water vapors.
It stated that all matter was made up of small indivisible particles known as atoms. This law explains that the sum of all partial pressures of gas in a mixture should equal the barometric pressure. At high pressure the volume occupied by a gas becomes significant when compared to the free space between particles.
Daltons Law of Partial Pressures or Daltons Law states that the total pressure of a gas in a container is the sum of the partial pressures of the individual gases in the container. Daltons Law plays a. Daltons law is also known as the law of partial pressure or Gibbs-Dalton law rarely.
The Law of Partial Pressure also known as Daltons law states that the pressures of all the gases in a mixture will add up to the total pressure of the gas solution. Daltons law of partial pressures is a gas law which states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures exerted by each individual gas in the mixture. Daltons law is valid for ideal gases.
Here is a worked example problem showing how to. Daltons law is an ideal gas law. If youve heard of a therapeutic oxygen tent you are seeing Daltons law of partial pressure put to work.
The law describes the relationship between the total pressure of a mixture of non-reacting ideal gases and the partial pressures of each individual component. Daltons law states that the partial pressure of a gas in a mixture is the pressure that gas would exert if it occupied the total volume of the mixture. In other words all the.
The total atmospheric pressure is the sum of the partial pressures of each gas. The partial pressure of each gas is the pressure at which each gas would exert if it occupied alone the volume at the same temperature. Simply put all of the gases in the Earths atmosphere exert individual pressures that add up to that 147 pounds per square inch psi.
The total pressure of a mixture of gases is equal to the sum of the partial pressures of the individual gases in the mixture. Dawltonz the pressure exerted by a mixture of nonreacting gases is equal to the sum of the partial pressures of the separate components. Daltons Law or the Law of Partial Pressures states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the gases in the mixture.
Daltons law of partial pressure is used to calculate pressure of dry gas when gas is collected over water. This empirical law was observed by John Dalton i. Change the concentration of airs gases for example making it.
Deviations From Daltons Law. Daltons law is a principle used in chemistry to predict the concentration of mixed gases in terms of pressure. Explanation Based on the kinetic theory of gases a gas will diffuse in a container to fill up the space it is in and does not have any forces of attraction between the molecules.
Daltons law - chemistry and physics law stating that the pressure exerted by a mixture of gases equals the sum of the partial pressures of the gases in the mixture. When gas is collected over water the gas also contains water vapour due to evaporation. For example the total pressure exerted by a mixture of two gases A and B is equal to the sum of the individual partial pressures exerted by gas.
Thus the ideal gas law tells us that the partial pressure of hydrogen in the mixture is. P moist P dry f aqueous tension. In Chemistry and Physics Daltons law also called Daltons law of partial pressures states that in a mixture of non-reacting gases the total pressure exerted is equal to the sum of the partial pressures of the individual gases.
Also known as Daltons law of partial pressure it states that the sum of exerted pressure of the whole mixture of gases is equal to the sum of all pressures in the mixture. Chemist John Dalton was born September 6 1766 in Eaglesfield England. Heres the formula of daltons law.
P Total P 1 P 2 P 3.

I Dalton S Law A The Total Pressure Of A Mixture Of Gases Equals The Sum Of The Pressures Each Gas Would Exert Independentl Dalton S Law Gas Laws Ideal Gas Law

Dalton S Law Of Partial Pressures Classwork Homework In 2021 Dalton S Law Classwork Ideal Gas Law

Gas Laws Dalton S Law Practice Problems Printable And Digital In 2021 Dalton S Law Law Dalton

Gas Laws Of Respiratory Physiology Boyle S The Pressure Of A Given Quantity Of Gas Is Inversely Proportional To Physiology Dalton S Law Anatomy And Physiology

Gas Laws Dalton S Law Practice Problems Printable And Digital Dalton S Law Teaching Chemistry Chemistry

Gas Law Quiz Dalton S Law Graham S Law Ideal Gas Law Dalton S Law Ideal Gas Law Science Chemistry

Energy Work And Heat Notes Internal Energy Chemistry Notes Chemistry

Daltons Law Of Partial Pressures Easy Science Dalton S Law Pressure Law Easy Science

Charles Law Boyle S Law Dalton S Law Avogadro S Law High School Science Teacher Teaching Chemistry Chemistry Classroom

Dalton S Law Of Partial Pressure Ideal Gas Equation Gases And Kinetic Molecular Theory Chemistry Dalton S Law Anatomy And Physiology Physical Chemistry

Dalton S Law Of Partial Pressureslaw Dalton Pressures Partial Dalton S Law Thermodynamics Partial

Chemistry Tutorial Dalton S Law Of Partial Pressures Dalton S Law Chemistry Dalton

Daltons Law Freediving Dalton S Law Physiology Respiratory System

Dalton S Law Chemical Physics Equation Example Diagram Dalton S Law Physics Gas Laws

Laws Of Science And Medicine Dalton S Law Gas Laws Chemistry Avogadro S Law

Learn Quiz On Kinetic Molecular Theory Of Gases Chemistry Quiz 240 To Practice Free Chemistry Mcqs Questio College Chemistry Chemistry This Or That Questions

Charles Law Boyles Law Charles Law Henryslaw Daltons Law Science Chemistry Boyle S Law Science Anchor Charts

Partial Pressure And Total Pressure Dalton S Law Chemistry Class

Dalton S Law Of Partial Pressures Explained Dalton S Law Medical Anatomy Respiratory Therapy
Comments
Post a Comment